Wheel Change Kit
| FC200829 | |
| Ultrasound | |
| GE HealthCare | |
| GE HealthCare | |
Insira o seu número de aprovação e submeta para adicionar item (s) no carrinho.
Please enter approval number
OR
Não sabe o seu número de aprovação? Ligue para 800-437-1171
Enter opt 1 para os primeiros três avisos, e tenha o seu ID do Sistema disponível.
Se você adicionar item (s) no carrinho e enviar seu pedido sem o número de aprovação
, a GE irá contactá-lo antes que o seu pedido
seja confirmado para entrega.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Visão geral do produto
- The Wheel Change Kit is used in Ultrasound and other medical purposes as applicable. It is a kit consisting of wooden wedge and bevel edge board. It is used to transport, hold, move or support an object. The bevel edge board has an angle formed at the edge of the wood, where the higher end has 45 mm thickness and lower end has 3 mm thickness. The wooden wedge has inclined position, where one end is higher than another end. The thickness of the higher end position is 119.5 mm and the thickness of the opposite end is 25.5 mm. They can easily fix and portable. It is made from the solid pinewood material, which possess malleability, fluidity, soft and white in color. Also has shrinking resistant and swelling resistant. It is manufactured using high precision techniques which helps to achieve a good surface finish as well as good product consistency. The GE product is an Innovation and a state of the art technology which fits well into versatile customer needs. The part is diligently designed for superior Performance and reliability. It is securely packed inside a High-quality packing box to avoid physical damage during transit and labeled with details about the product, Quality Assurance (QA) seal and shipment details.
Produtos Compatíveis
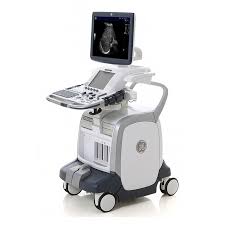
LOGIQ E9
The LOGIQ* E9 is a versatile general imaging system that helps meet a wide variety of general imaging needs. As healthcare evolves and ultrasound exams become more in demand, you need the ability to adapt to the changing environment with an ultrasound system that gives you flexibility, ease of use, speed and reliability.
The LOGIQ E9 can help you:
Deliver extraordinary image quality on a broad spectrum of patient body types.
Visualize blood flow without the limitations of Doppler
Enhance your workflow.
Integrate real-time ultrasound with previously acquired CT, MR, PET, or ultrasound images.
Visually track your position during a scan.
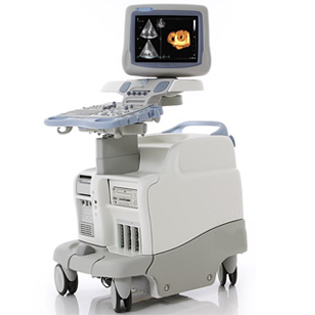
Vivid 7
The Vivid 7/Vivid 7 PRO ultrasound unit is a high performance digital ultrasound imaging system.The system provides image generation in 2D (B) Mode, Color Doppler, Power Doppler (Angio), M-Mode, Color M-Mode, PW and CW Doppler spectra, Tissue Velocity imaging and Contrast applications. The fully digital architecture of the Vivid 7/Vivid 7 PRO unit allows optimal usage of all scanning modes and probe types,throughout the full spectrum of operating frequencies.The Vivid 7 Dimension gives clinicians a better way to convey their findings to other cardiologists, referring physicians,EP physicians and patients. Now, cardiac anatomy,synchronicity and viability can be clearly communicated in imaging formats that are more familiar for your clinical partners, thus easier to understand.
• Real-time 4D imaging – provides more cardiac information to help clinicians better communicate the heart’s structure and function.
• 4D Tissue Synchronization Imaging (TSI) – propels Tissue Velocity Imaging (TVI) to the next level by taking three simultaneous planes – from a single heartbeat at high frame rates – to create a flexible, dynamic 4D model with quantitative measurements to better communicate cardiac dyssynchrony.
• Bull’s-eye report formats and TSI surface mapping –communicate cardiac dyssynchrony in a visual display that should be more familiar to EP physicians.
• Blood Flow Imaging (BFI) – new vascular imaging mode gives clinicians a better understanding and delineation of directional blood flow in vessels.
• Seamless measurement integration – allows you to efficiently calculate ejection fraction and volumes from tri-plane images gathered from the same heartbeat.