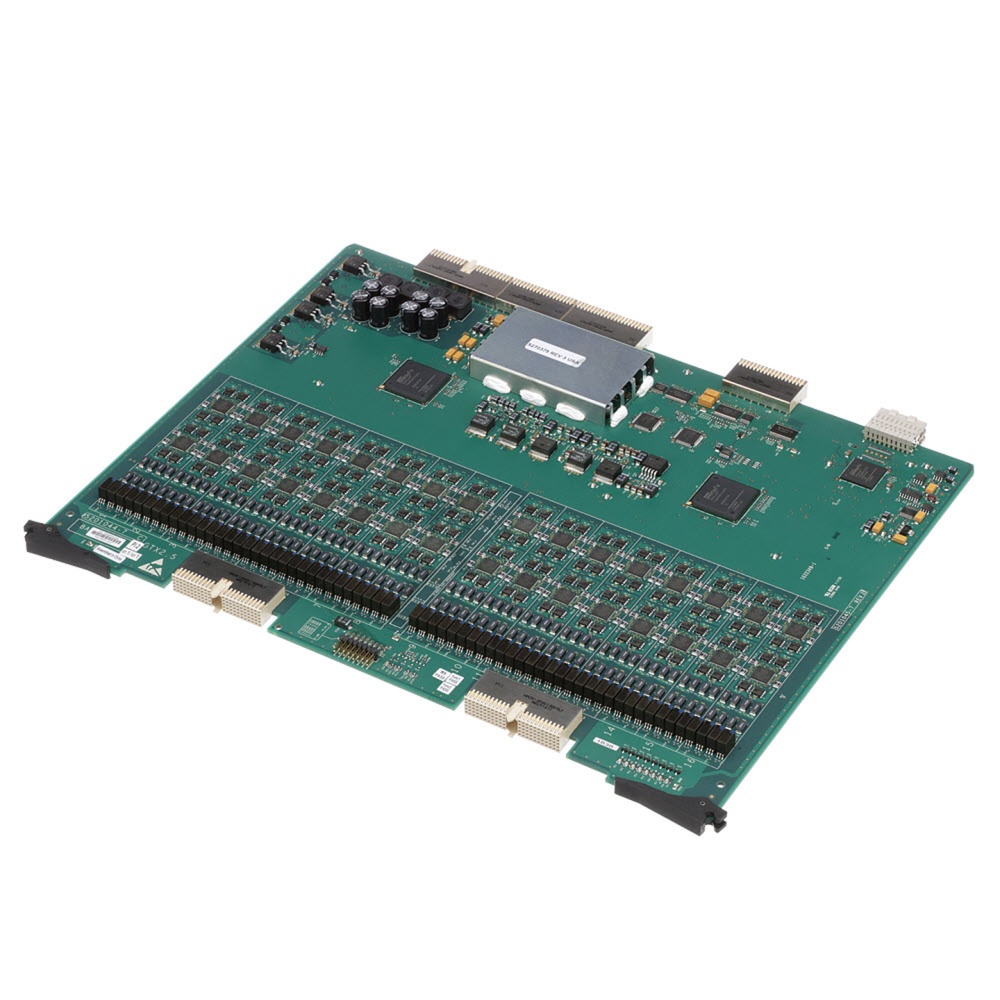
Ichiro Global Transmit Board GTX 2.5
| 5201044-7-R | |
| Ultrasound | |
| GE HealthCare Parts | |
| GE HealthCare | |
Insira o seu número de aprovação e submeta para adicionar item (s) no carrinho.
Please enter approval number
OR
Não sabe o seu número de aprovação? Ligue para 800-437-1171
Enter opt 1 para os primeiros três avisos, e tenha o seu ID do Sistema disponível.
Se você adicionar item (s) no carrinho e enviar seu pedido sem o número de aprovação
, a GE irá contactá-lo antes que o seu pedido
seja confirmado para entrega.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Visão geral do produto
- The Ichiro Global Transmit Board GTX 2.5 is used in Ultrasound and many other medical systems. It is comprised of a resistor, capacitor, inductor, ferrite bead, diode rectifier, memory, IC, regulator, connector, ferrite chip, converter and transformer. The purpose of the GTX board is to provide the transmitter channel hardware and function for the Ichiro/Jiro beamformer system. Each GTX board in the system contains 64 transmit channels. The Ichiro/Jiro system is designed to contain a maximum of four GTX boards for a total of 256 channels. When populated with three GTX boards for a total of 192 channels the system configuration is referred to as âIchiroâ. When populated with four GTX boards (256 channels) the configuration is called âJiroâ. It is manufactured using high precision techniques which helps to achieve a good surface finish as well as good product consistency. The part is diligently designed for high Performance and reliability. It is securely packed inside a high quality packing box to avoid physical damage during transit and labeled with details about the product, Quality Assurance (QA) seal and shipment details.